In this post, we investigate the importance of verb choice within Quality Management System (QMS) procedures and work instructions.
We will look at:
- What the important verbs are and when to use them
- Give helpful tips for reviewing procedures to ensure you do not miss any of the important requirements
- Introduce some practices to avoid and provide solutions to consider
All this and more to help you have a great result during your next audit or inspection!
To get us started let’s look at what ISO 13485 has to say about the verbs used in the quality management system standard.
The authors of ISO 13485 have included a clarification of concepts section which includes, among other topics, a description of their use of the following verb forms used throughout the standard:
- Shall (Requirement)
- Should (Recommendation)
- May (Permission)
- Can (Possibility or Capability)
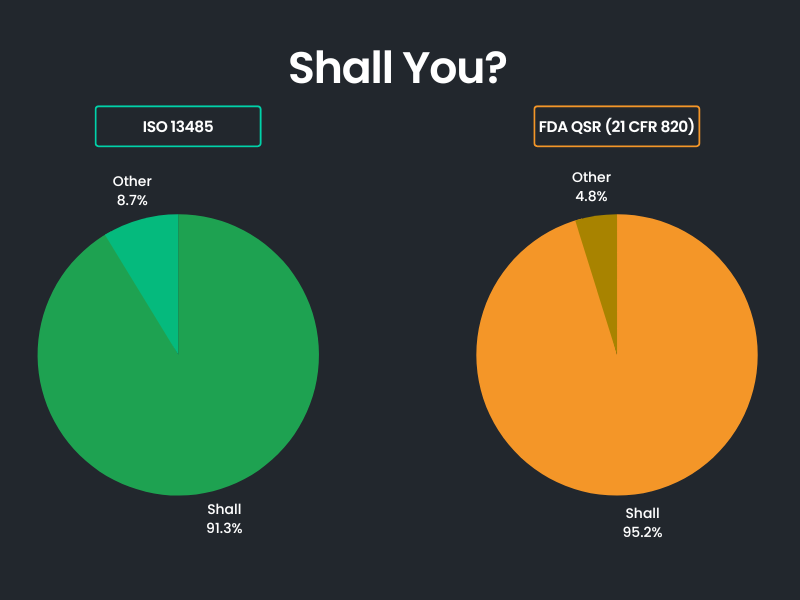
It should come as no surprise to you that overwhelmingly “Shall” is used throughout the ISO 13485 standard with just a few instances of the other verbs listed above being used.
A similar picture is painted upon review of FDA’s Quality System Regulation (21 CFR 820). See the breakdown in the graphic which shows the high frequency of the use of “Shall” in the main body of the documents.
What shall you do?
Everywhere a standard or regulation uses “shall” you must demonstrate within your quality management system (QMS) that the requirement is documented. You must pay close attention when you see “shall” and mirror this meaning within your QMS documentation.
This is not the time to try to sneak in one of the less definite verbs to attempt to give yourself an excuse if you fail to show evidence of meeting the requirement during the audit.
But this is not to say that you should make every verb a “Shall” when “Should”, “May”, or “Can” is appropriate. More on this topic in a minute.
HELPFUL TIPS
- Use the Find feature (Ctrl F) to find all the instances of “Shall”, “Should”, “May”, and “Can” within the standard or regulation document you need to comply with to produce a checklist of requirements you do not want to miss along with areas where other verbs may be acceptable.
- As you review your procedure use the Find feature (Ctrl F) to find instances of “Shall”, “Should”, “May”, “Can”, and other similar verbs to ensure the right intent is being used and it aligns with the checklist you created in the tip above.
When and why are the “Other Verbs” used within ISO 13485 and FDA’s QSR?
Should
In the QSR this verb is not used at all. In ISO 13485 it is used just once in the definition of Manufacturer because regulatory requirements may vary depending on competent authority.
May
Used just a few times within the definitions of ISO 13485, again since regulatory requirements vary depending on the competent authority. Within the QSR “May” is used a few times to indicate Possibility or Capability like how “Can” is defined in ISO 13485.
Can
This verb is used in ISO 13485 to show compliance with the standard is voluntary, to refer to other standards for further information, and within several definitions. It is also used in a few places within the main body where certain applications or scenarios have the capability to impact quality. “Can” is used just a few times within the QSR in a comparable manner.
Things to Watch Out For or Avoid
Well-intentioned Authors
The well-intentioned author may be tempted to include all the “nice to have” best practices or recommendations for a given process within the procedure without intending to be held to discrete compliance.
CAUTION: If you use “shall” in the procedure, even if the process is not required according to the standard or regulation, auditors are likely to hold you to it and expect to see evidence that it is happening.
Often these are best practices related to helping things run more smoothly or efficiently. Including this information can be especially important to how an organization runs, but if not strictly required to carry out the intent of a requirement within the standard or regulation you should carefully consider whether it is best to include it.
If it is truly a recommendation that is not required to carry out the intent of a requirement, then it may be better to leave it out of the procedure. Alternatively, if the decision is made to include it then it should be carefully referred to as guidance or a similar term.
This is where it may be appropriate to utilize “Should”, “May”, or “Can”. However, you do want to use caution because even if you identify it as a recommendation an auditor may see it as necessary to carry out a requirement within a standard or regulation and you’ll be in for a fight.
Be Practical (Risk-Based)
Being overly prescriptive in the definition of a process can often be a trap. The best approach may not be to have extremely detailed instructions for every step depending on the risks related to the process.
Where the associated risks are low, and the standard or regulation does not prescribe a particular approach, then it may be better to leave the process open to some variation especially in smaller organizations that rely on a few qualified and trained individuals.
In general, as the complexity of the organization, the number of people involved, and the risks involved increase, the prescriptive nature of the process may need to be increased as well. But you should always be careful not to take it too far and implement something that is so detailed that people cannot understand how to use it or consistently apply it.
“If you can’t explain it to a six year old, you don’t understand it yourself.”
― Albert Einstein
You should only plan to include best practices as “shall” statements if your organization can truly stand behind the process and it is appropriately staffed to carry it out consistently. Otherwise, if it is important for your organization then only include these statements as guidance if you have a compelling argument for why they are not strictly required to carry out a requirement of the standard or regulation.
Summary
A terrific way to avoid receiving an easy nonconformance observation from an auditor is to pay attention to the use of which verbs are used and in what situations within your procedures and work instructions.
Follow my tips for using the Find (Ctrl F) function within software programs for identifying the important verbs to ensure at a minimum all the “shalls” are covered consistent with the intent of the standard or regulation.
Finally, always apply a risk-based practical approach to your quality management system procedures and balance your best intentions with the current capabilities and needs of your organization.
